The Kolisko Validation™ Method: Improving the Quality Control of Homeopathically Potentized OTC Drugs
Razvan “Ross” Rentea MD, Mark Kamsler MD
Summary:
We describe a standardized biological test, the Kolisko Validation™ method, which allows validation i.e. verification of effective activity of homeopathically potentized substances.
Only potentized substances that have been proven active in the biological (living) model are then included in the finished products that become commercially available.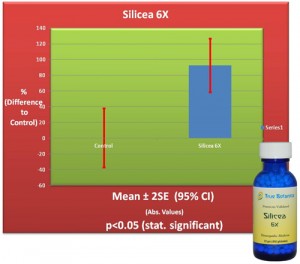
Potencies that are no different in their effectiveness than the untreated control medium are then eliminated from further use.
To our knowledge this is the first time that such an objective, statistically analyzable, “Potency Validation” test is included in the quality control process of manufacturing of homeopathic/anthroposophic products. This could constitute an additional step in assuring the consumer that the product containing an ultra-high dilution is not “just water/just sugar pills.”
Additionally, the Kolisko Validation™ test also allows a more objective interpretation of the potencies of each substance (is a potency a low or high potency, etc.)
It is hoped that ultimately the resulting potency validated homeopathic/anthroposophic medications will emerge as clinically more effective.